-
 Home
Home
-
 News
News
Latest Educational News Stories
Daily update of all national, international news, picture stories, college / university announcements and educational events.
-
 Colleges
Colleges
Pakistan's Largest Database of Colleges and Universities
Explore Largest Directory of Private and Govt. Colleges, Universities and find best institute for your future Education.
-
 Courses
Courses
-
 Admission
Admission
-
 Lectures
Lectures
-
 Online Test
Online Test
Short Question
- 9th Class Physics Short Questions
- 9th Class Chemistry Short Questions
- 9th Class Math Short Questions
- 9th Class Biology Short Questions
- 9th Class Computer Short Questions
- 9th Class English Short Questions
- 10th Class Physics Short Question
- 10th Class Chemistry Short Question
- 10th Class Math Short Question
- 10th Class Biology Short Question
- 10th Class Computer Short Question
- 10th Class English Short Question
-
 Past Papers
Past Papers
-
 Date Sheets
Date Sheets
-
 Results
Results
Exam Results 2024
Check online Results 2024 Matric Inter BA BSc B.Com MA MSc M.Com CSS PCS MCAT ECAT of all educational boards and universities in Pakistan
-
 Study Abroad
Study Abroad
Study Abroad Programs and Opportunities for Pakistani Students
Explore free study abroad search to find programs, consultants, events to study in USA, UK, Australia, China, Malaysia and many others.
-
 Jobs
Jobs
-
 Tutors
Tutors
-
 More
More
-
 Apps
Apps
All the fresh passionate candidates who have just completed their intermediate studies and now are looking forward towards engineering field must need to clear Engineering College Admission Test (ECAT). Now the question arises how and from where we have to prepare for the test. So the answer is very simple ilmkidunya.com provides an online test preparatory facility to the students. Now all the aspirants can prepare themselves by sitting at home and also free of cost. Just after pressing Start button the students will get online MCQs test that will prepare them for the ECAT Chemistry.
ECAT a pre-requisite for getting admission in medical institutes and universities is a multiple choice questions based test with a total of 100 MCQs out of which 30 MCQs will be included from Chemistry section as per the test structure approved by UET.
ECAT Chemistry MCQ's Test For Full Book
Try The ECAT Chemistry MCQ's Test For Full Book
-
Total Questions30
-
Time Allowed30
Activation energy is the difference of energy between the energy of the reactant and
A colourless liquid, at room temperature reacts with soda lime to form sodium salt of carboxylic acid and ammonia gas. The liquids is
The elements in which d or f-orbitals are incomplete are called
In the rate equation when the concentration of reactants are unity, then rate is equal to
The oxidation number of each element of group ll-A is
Value of rate constant k is specific for a reaction, and varies from reaction to reaction. The value of k of a reaction changes with
5-d series is in the period :
Hybridized in oxygen is:
The chemical reactivity of glass is reduced by the use of
The apex angle of the folded filter paper is slightly greater is termed as:
Atmosphere thickness is (in km) around the earth:
Formic acid is obtained when
When alkyl is treated with chlorine in the presence of sunlight
The majority of reactions which give stable products are:
The Mn3+has ______ color
Use of ethanol as:
The reaction of acetaldehyde with HCN followed by hydrolysis gives a product which exhibits
Which one of the following equations represent the reaction that occurs when calcium nitrate is heated strongly
Saturated solution of a solid is prepared at a constant temperature. 100 cm3of this saturated solution is evaporated in a china dish. The mass of the residue is called
The concentration of reactants is increased by x, then equilibrium constant K becomes
DDT is
Hydrolysis of an ester gives a carboxylic acid which on Kolbe's electrolysis yields ethane. the easter is
Acetylene when treated with 10% H2SO4in the presence of HgSO4adds one molecule of water to form
Sodium belongs to block of periodic table:
All the following decompose easily on heating to give oxygen except
The principle quantum number describes
Which has sweetish taste?
Williamson's synthesis is used to prepare
Simplest aromatic compound is
CH3CH2COOH is also named as:
Here is List Of Chapter Wise Tests
| Ch. # | Test Name | MCQs Available | PDF File | Launch Test |
|---|---|---|---|---|
| 1 | ECAT Chemistry Chapter 1 Basic Concepts | 109 | Download PDF | Launch Test |
| 2 | ECAT Chemistry Chapter 2 Experimental Techniques in Chemistry | 58 | Download PDF | Launch Test |
| 3 | ECAT Chemistry Chapter 3 Gases | 66 | Download PDF | Launch Test |
| 4 | ECAT Chemistry Chapter 4 Liquids & Solids | 60 | Download PDF | Launch Test |
| 5 | ECAT Chemistry Chapter 5 Atomic Structure | 166 | Download PDF | Launch Test |
| 6 | ECAT Chemistry Chapter 6 Chemical Bonding | 116 | Download PDF | Launch Test |
| 7 | ECAT Chemistry Chapter 7 Thermo Chemistry | 55 | Download PDF | Launch Test |
| 8 | ECAT Chemistry Chapter 8 Chemical Equilibrium | 159 | Download PDF | Launch Test |
| 9 | ECAT Chemistry Chapter 9 Solutions | 164 | Download PDF | Launch Test |
| 10 | ECAT Chemistry Chapter 10 Electrochemistry | 162 | Download PDF | Launch Test |
| 11 | ECAT Chemistry Chapter 11 Reaction Kinetics | 112 | Download PDF | Launch Test |
| 12 | ECAT Chemistry Chapter 12 Periodic Classification of Elements and Periodicity | 35 | Download PDF | Launch Test |
| 13 | ECAT Chemistry Chapter 13 S-Block Elements | 51 | Download PDF | Launch Test |
| 14 | ECAT Chemistry Chapter 14 Group IIIA & IVA Elements | 170 | Download PDF | Launch Test |
| 15 | ECAT Chemistry Chapter 15 Group IV-A and VI-A Elements | 58 | Download PDF | Launch Test |
| 16 | ECAT Chemistry Chapter 16 The Halogens and The Noble Gases | 64 | Download PDF | Launch Test |
| 17 | ECAT Chemistry Chapter 17 Transition Elements | 160 | Download PDF | Launch Test |
| 18 | ECAT Chemistry Chapter 18 Fundamental Principles of Organic Chemistry | 158 | Download PDF | Launch Test |
| 19 | ECAT Chemistry Chapter 19 Aliphatic Hydrocarbons | 202 | Download PDF | Launch Test |
| 20 | ECAT Chemistry Chapter 20 Aromatic Hydrocarbons | 61 | Download PDF | Launch Test |
| 21 | ECAT Chemistry Chapter 21 Alkyl Halides | 165 | Download PDF | Launch Test |
| 22 | ECAT Chemistry Chapter 22 Alcohols, Phenols and Ethers | 159 | Download PDF | Launch Test |
| 23 | ECAT Chemistry Chapter 23 Aldehydes and Ketones | 140 | Download PDF | Launch Test |
| 24 | ECAT Chemistry Chapter 24 Carboxylic Acid Online Test | 174 | Download PDF | Launch Test |
| 25 | ECAT Chemistry Chapter 25 Macromolecules | 167 | Download PDF | Launch Test |
| 26 | ECAT Chemistry Chapter 26 Common Chemical Industries in Pakistan | 30 | Download PDF | Launch Test |
| 27 | ECAT Chemistry Chapter 27 Environmental Chemistry | 118 | Download PDF | Launch Test |
| 0 | 109 | Download PDF | Launch Test | |
| 0 | 131 | Download PDF | Launch Test | |
| 0 | 31 | Download PDF | Launch Test | |
| 0 | 31 | Download PDF | Launch Test | |
| 0 | 30 | Download PDF | Launch Test | |
| 0 | 76 | Download PDF | Launch Test | |
| 0 | 26 | Download PDF | Launch Test | |
| 0 | 29 | Download PDF | Launch Test |
Top Scorers of ECAT Chemistry MCQ's Test For Full Book
-
U Umme Jaan 11 - Dec - 2024 10 Min 39 Sec 80/120 -
A Amina Amjad 07 - Dec - 2024 08 Min 04 Sec 75/120 -
A Asif Javed 18 - Dec - 2024 26 Min 48 Sec 75/120 -
A Ahazz Khan 19 - Dec - 2024 10 Min 52 Sec 70/120 -
A Abdul Hadi 19 - Dec - 2024 08 Min 57 Sec 65/120 -
M Maria Shaban 28 - Nov - 2025 05 Min 24 Sec 60/120 -
N Noor Fatima 12 - Jan - 2025 08 Min 46 Sec 60/120 -
H Hira Ghazanfar 20 - Dec - 2024 04 Min 43 Sec 55/120 -
H Hamza Waseem 21 - Dec - 2024 10 Min 01 Sec 55/120 -
A Ayesha Asif 19 - Dec - 2024 08 Min 15 Sec 50/120 -
H HMUsman HMUsman 25 - Nov - 2024 13 Min 00 Sec 50/120 -
M Muhammad Haseeb 20 - Nov - 2024 25 Min 56 Sec 50/120 -
M M. Umer 27 - Oct - 2025 07 Min 05 Sec 40/120 -
N notghouri playz 20 - Dec - 2024 12 Min 34 Sec 40/120 -
A Abdullah Liaqat 05 - Dec - 2025 00 Min 02 Sec 36/120
| Sr.# | Question | Answer |
|---|---|---|
| 1 | Tetrahedral lead added to petrol act as |
A. Auto catalyst
B. Inhibitor
C. Activator
D. All of these
|
| 2 | Aldehydes give reactions : |
A. Oxidation and reduction
B. Base-catalysed nucleophilic
C. Acid catalysed nucleophilic
D. All of these
|
| 3 | Four d-orbitals contain four lobes while fifth contains only two lobes the orbital is |
A. dxy
B. dxz
C. dz2
D. dx2- y2
|
| 4 | Neutron was discovered by: |
A. Chadwick.
B. Bohr.
C. Rutherford.
D. Plank.
|
| 5 | Transition elements have valence electrons in |
A. s-orbital
B. p-orbital
C. d-orbital
D. f-orbital
|
| 6 |
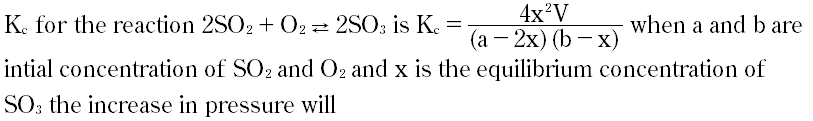
|
A. Shift reaction toward forward direction
B. Shift reaction backward
C. Lower the value of Kc
D. No change in reaction
|
| 7 | By simply reacting Grignand's reagent with water we get |
A. An alkane
B. Higher alkane
C. An alkene
D. An alkyne
|
| 8 | 3.01 x 1022Ag+ions is present in |
A. 85 grams AgNO3
B. 0.85 g AgNO3
C. 8.5 g AgNO3
D. 18.5 g AgNO3
|
| 9 | Which heavy metals do not have any safe limits |
A. As
B. Hg
C. Cr
D. All of these
|
| 10 | With increasing principle quantum number, the energy difference between adjacent energy levels in H atom |
A. Decreases
B. Increases
C. Remains constant
D. Decreases for low value of Z and increase for higher value of Z
|
| 11 | When alcohol reacts with concentreated H2SO4intermediate compound formed as |
A. carbonium ion
B. alkoxy ion
C. alkyl hydrogen sulphate
D. non of these
|
| 12 |

|
A. 1
B. 10
C. 5
D. 0.33
|
| 13 | The strongest forces are |
A. Debye forces
B. London dispersion forces
C. Dipole-dipole attraction
D. Hydrogen
|
| 14 | The bond angle depends upon the |
A. Types of bonds
B. Number of bonds
C. Non-bonding electron pairs
D. All of the above
|
| 15 | The standard enthalpy change in the formation of 1 mole of a compound from its elements in their standard physical states is |
A. Enthalpy of formation
B. Enthalpy of atomization
C. Enthalpy of neutralization
D. Internal energy change
|
| 16 | Hydrolytic reaction of fats by causic soda is known as |
A. Acetylation
B. Carboxylation
C. Esterification
D. Saponification
|
| 17 | The freezing mixture used in ice cream machine consists of ice and |
A. NaCI
B. KCI
C. MgCI2
D. NaNO3
|
| 18 | High purity copper metal is obtained by |
A. Carbon reduction
B. Hydrogen reduction
C. Electrolytic reduction
D. Thermite reduction
|
| 19 | Homogenous catalysis is that in which catalyst and reactants are in same phase. Which one of the following reaction is a homogenous catalysis |
A.
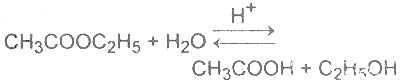
B.
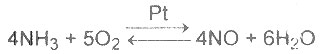
C.
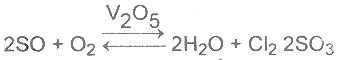
D.
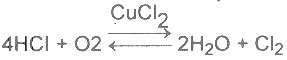
|
| 20 | The oxidation number of chromium in K2Cr2O7is |
A. 14
B. 12
C. 6
D. None of these
|
| 21 | Azeotropic mixture of HCl and water has |
A. 48% HCl
B. 22.2% HCl
C. 36% HCl
D. 20.2% HCl
|
| 22 | Benzene gives reactions generally: |
A. Electropholic subsitution
B. addition
C. synthesis
D. addition and electropholic subsitution
|
| 23 | Macromolecules or polymers are large molecules built up from small molecules called monomers. This hypothesis put forward by |
A. Schrodixger
B. Standinger
C. Lewis
D. Newton
|
| 24 | Aldelydes are the oxidation product of |
A. P-alcohols
B. s-alcohols
C. ter-alcohols
D. carboxylic acids
|
| 25 | Which of the following geometry is associated with the compound in which the central atom assumes sp3d hybridization? |
A. Planar
B. Pyramidal
C. Angular
D. Trigonal bipyramidal
|
| 26 | Which one of the following compounds does not have the empirical formula CH2O? |
A. Ethanoic acid, CH3CO2H
B. Ethanol, CH3CH2OH
C. Glucose, C6H12O6
D. Methanal, HCHO
|
| 27 | When ammonia is heated with cupric oxide, a molecule of ammonia will |
A. Gain 3 electrons
B. Lose 3 electrons
C. Gain 2 electrons
D. Lose 2 electrons
|
| 28 | The OH group present in acids may be replaced by CI atom on treatment with |
A. PCI5
B. SOCI2
C. Both of them
D. None of the above
|
| 29 | The process in which one s and two p orbitals mix up with each other is called |
A. Sp-hybridization
B. Sp2-hybridization
C. Sp3-hybridization
D. Dsp2-hybridization
|
| 30 | Hydrocarbons contain |
A. C and S only
B. C and H only
C. C, H, and O only
D. C, H, O and N only
|
Syllabus recommended by UET for Chemistry subject is as under: Part 1 Basic Concepts, Experimental Techniques in Chemistry, Gases, Liquid and Solids, Atomic Structure, Chemical Bonding, Thermo chemistry, Chemical Equilibrium, Solutions, Electrochemistry, Reaction Kinetics B. Part 2 Periodic Classification of Elements and Periodicity, S-Block Elements, Group III-A and Group VI-A Elements, Group IV-A and Group VI-A Elements The Halogens and the Noble Gases, Transition Elements, Fundamental Principles of Organic Chemistry, Aliphatic Hydrocarbons, Aromatic Hydrocarbons, Alkyl Halides, Alcohols, Phenols and Ethers, Aldehydes and Ketones, Carboxylic Acids, Macromolecules, Common Chemical Industries in Pakistan Environmental Chemistry
Attempting the test below will give you a real time experience of practicing ECAT test. It will improve your preparation to get high marks in the test and also will help you in getting admission in your desired engineering university/college.













